Methanol to hydrogen has become one of the most practical ways to supply clean hydrogen where pipelines and high-pressure cylinders are not convenient. Many companies want hydrogen for fuel cells, steel processing, electronics, or chemical production. However, storing and transporting hydrogen remains expensive and risky. Cylinders require strict safety control. Tube trailers add logistics costs. Infrastructure is still limited.
Because of these challenges, more engineers now choose it to conversion. Methanol acts as a liquid hydrogen carrier. It stores easily, ships safely, and uses existing fuel infrastructure. Then, an on-site system converts methanol into hydrogen whenever needed.
This approach creates hydrogen on demand, reduces transport risks, and lowers overall cost. In this guide, we explain how methanol to hydrogen works, compare the main technologies, break down equipment and costs, and help you select the right system for your project.
Why Methanol to Hydrogen Is a Smart Hydrogen Supply Strategy
First, methanol is liquid at room temperature. So you can store it in normal tanks.
Second, methanol has high energy density. One truck can deliver large amounts of hydrogen potential.
Third, you can install a compact methanol to hydrogen conversion unit directly at your site.
As a result, you get:
• No hydrogen cylinders
• No long-distance hydrogen transport
• Lower safety risk
• Continuous gas supply
• Flexible production
This is why many methanol to hydrogen fuel cell projects and distributed energy systems now adopt on-site reforming.
Main Chemical Routes for Methanol to Hydrogen Conversion
Several reaction paths exist. Each one fits different needs.
1. Methanol Steam Reforming (MSR) – Most Mature
Reaction:
CH₃OH + H₂O → CO₂ + 3H₂
This is the most widely used its technology.
Features
• High hydrogen yield
• Stable operation
• Good efficiency
• Mature catalysts
• Ideal for continuous industrial use
Most commercial plants use MSR.
2. Partial Oxidation (POX)
CH₃OH + 0.5O₂ → CO₂ + 2H₂
• Very fast start
• Simple reactor
• Lower hydrogen yield
Good for quick-response systems.
3. Methanol Cracking
CH₃OH → CO + 2H₂
• High CO concentration
• Requires extra purification
Less common today.
4. Autothermal Reforming (ATR)
Combines MSR + POX.
• Self-balanced heat
• Medium efficiency
• Good for dynamic loads
Quick Comparison Table
| Route | Temp | H₂ Yield | Advantages | Limits |
|---|---|---|---|---|
| MSR | 200–300°C | High | Mature, efficient | Needs heat |
| POX | 300–500°C | Medium | Fast start | Lower yield |
| Cracking | 300–400°C | Medium | Simple | High CO |
| ATR | 250–400°C | Medium-High | Energy balance | Complex |
For most factories, methanol to hydrogen (MSR) remains the best choice.
How Methanol to Hydrogen Works – Full Process Flow (MSR Example)
Let’s walk through a typical methanol to hydrogen conversion system.
Step 1 – Feed Preparation
Mix methanol with deionized water. Then vaporize the mixture.
Step 2 – Catalytic Reforming
The vapor enters a reactor with Cu/ZnO/Al₂O₃ catalyst.
Hydrogen forms quickly.
Step 3 – Water Gas Shift (WGS)
CO converts into CO₂.
This step increases hydrogen output and reduces impurities.
Step 4 – Gas Purification
Use:
• PSA (Pressure Swing Adsorption)
• or membrane separation
Hydrogen purity can reach 99.999% or higher.
Step 5 – Heat Recovery
Reuse waste heat to preheat feed.
This improves efficiency and reduces energy cost.
A well-designed plant can reach excellent thermal performance.
Core Equipment in a Methanol to Hydrogen System
A complete system includes:
• Reforming reactor (tubular or microchannel)
• Vaporizer
• Heat exchangers
• PSA purification unit
• Control system
• Safety system
• Catalyst modules
Catalyst Matters
Catalyst life affects cost directly.
Good catalysts offer:
• Long life
• Stable activity
• Easy replacement
• Lower energy demand
High-quality catalysts reduce downtime and improve ROI.
Commercial Applications of Methanol to Hydrogen
Today, this system supports many industries.
1.Distributed Hydrogen Stations
Produce hydrogen on-site for buses, trucks, and forklifts.
Perfect for fuel cell vehicles.
2.Steel & Metallurgy
Provide reducing gas for furnaces.
3.Electronics & Photovoltaics
Supply ultra-pure hydrogen for coating and semiconductor processes.
4.Chemical & Pharmaceutical
Portable hydrogen source for synthesis.
5.Renewable Energy Storage
Convert green methanol back into hydrogen when needed.
Economics: Is Methanol to Hydrogen Cost-Effective?
Cost drives most decisions. Let’s break it down.
CAPEX (Equipment Cost)
Typical ranges:
• 10 Nm³/h – small lab or pilot
• 100 Nm³/h – mid-scale factory
• 500+ Nm³/h – industrial station
Larger systems reduce unit cost.
OPEX (Operating Cost)
Main factors:
• Methanol price
• Electricity
• Water
• Catalyst replacement
• Maintenance
Simple Cost Example
Hydrogen cost ≈
(Methanol consumption + power + maintenance) ÷ hydrogen output
When methanol price is stable, this conversion often costs less than delivered hydrogen cylinders.
If you want a quick estimate, you can use our Hydrogen Calculator
It helps you calculate real production cost in minutes.
How to Choose the Right Methanol to Hydrogen System
Before buying, check:
• Required flow rate (peak & average)
• Purity target
• Space limitation
• Automation level
• Methanol supply stability
• Safety standards
Match the system size to your real demand.
Why Many Companies Choose HYVODA Methanol to Hydrogen Solutions
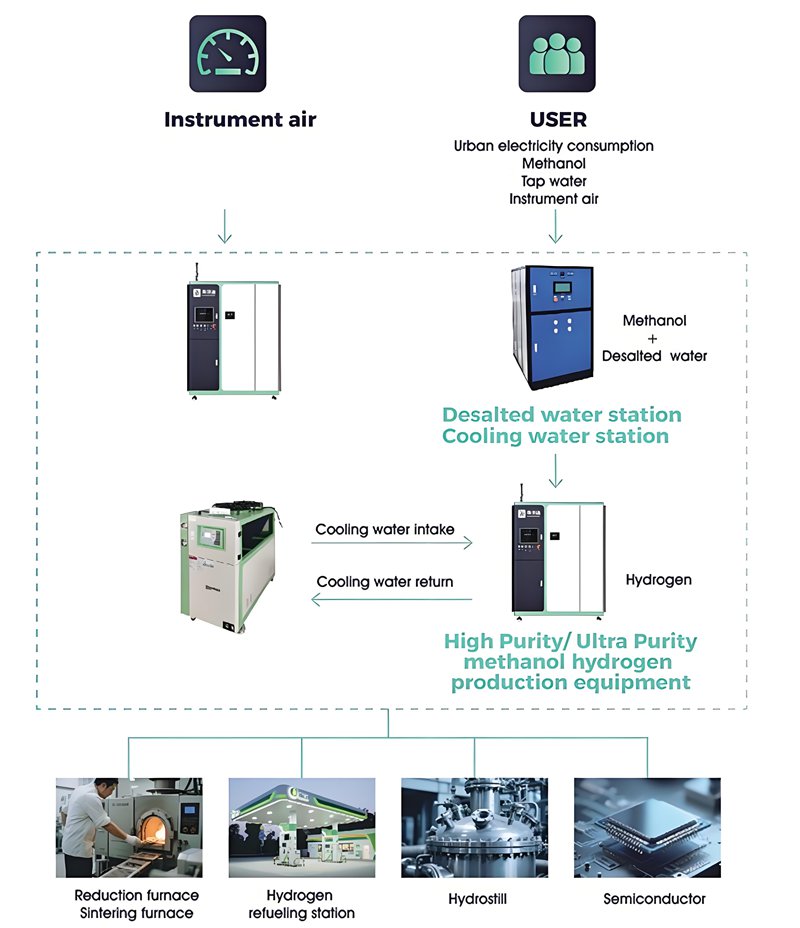
As a professional hydrogen energy company, HYVODA provides advanced methanol to hydrogen conversion systems for distributed and industrial use.
We supply complete skid-mounted solutions. Clients only need methanol, water, air, and electricity.
Key Advantages
1.Compact Skid Design
Highly integrated. Small footprint. Easy installation.
2.Wide Capacity Range
0.1–1000 Nm³/h
Flexible pressure, flow, and purity control.
3.High Efficiency
Advanced heat storage and waste heat recovery
Overall thermal efficiency > 80%
4.Smart & Safe
Explosion-proof design
Digital monitoring
Fully automatic unattended operation
5.Long-Life Catalyst
Self-developed catalyst
Service life > 24,000 hours
Room-temperature ignition reduces energy use by 80%
6.Proven Projects
Used in steel, electronics, pharma, and power sectors
Customers include Baowu Steel, HBIS, and China Power Investment.
So whether you need a small methanol to hydrogen fuel cell station or a large industrial plant, HYVODA delivers a reliable solution.
FAQ
Q1: What purity can methanol to hydrogen reach?
With PSA purification, purity can reach 99.999% or higher.
Q2: How fast can the system start?
Compact systems can start within 20–30 minutes depending on size.
Q3: How long does the catalyst last?
Typically over 24,000 hours.
Q4: Is it safe?
Yes. Systems include explosion-proof design, leak detection, and automatic shutdown.
Q5: What services does HYVODA provide?
We offer design, engineering, installation, commissioning, and long-term maintenance.
Final Thoughts
Methanol to hydrogen offers a practical, flexible, and cost-effective way to produce hydrogen on-site. It avoids transport problems and supports fuel cells, industry, and clean energy projects.
If you want reliable methanol to hydrogen conversion with high efficiency and smart control, HYVODA can help you design the right system.
Contact us today to discuss your project or calculate your hydrogen cost with our Hydrogen Calculator.
